Two cases of poorly differentiated chordoma were molecularly and immunologically profiled through the pediatric arm of the GSC’s POG program. Identification of a relatively high level of CD8+ T cells in the tumour microenvironment, in addition to expression of an immune checkpoint protein, lead to immune checkpoint inhibitor therapy for one patient. Analysis of RNA and DNA methylation profiles suggests that there’s a molecular subtype of immunologically hot pediatric tumours that could benefit from this treatment.
Chordomas are slow growing tumours found in the skull base and spinal column that are often resistant to chemotherapy and radiation. Of the three distinct subgroups, poorly differentiated chordoma (PDC) is the rarest and most likely to affect young people. PDC is characterized by expression of the tumour antigen, brachyury (an embryonic transcription factor encoded by the TBXT gene), and loss of INI1 (a chromatin remodelling factor subunit encoded by the SMARCB1 gene).
Two pediatric patients with PDC
A case report recently published in NPJ Precision Oncology describes the molecular analysis of two young chordoma patients in the pediatric arm of the GSC’s Personalized OncoGenomics program (POG), which to date has sequenced the tumours and germlines of 148 children with hard to treat cancers.
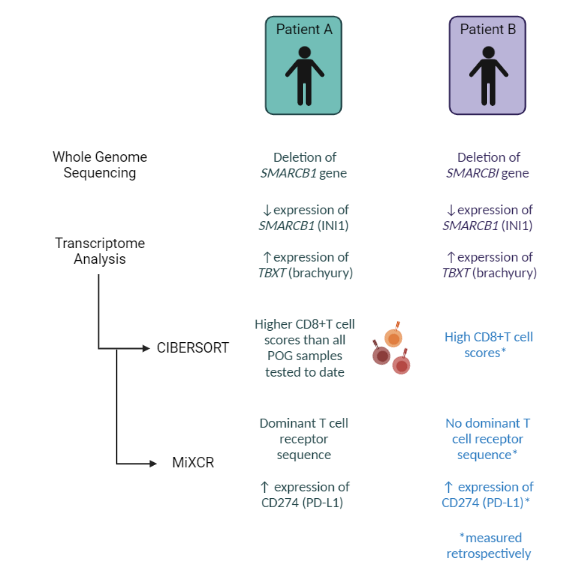
Patient A—the first patient described in the paper—was a previously healthy and active 8-year-old who presented with neck stiffness and pain. PDC was diagnosed by MRI, PET scan and pathology. The patient initially responded to chemotherapy but experienced recurrence and lung metastasis following radiation.
Patient B presented with a three-year history of headaches followed by increasing neck pain and limited range of movement. This patient was also diagnosed with PDC through imaging and histopathology, and initially responded to chemotherapy treatment.
Molecular and Immunological Analysis through POG
The results of POG analysis discussed at molecular tumour board meetings for each patient are summarized in the diagram below. CIBERSORT is a tool used to estimate the abundance of immune cell types in tumour samples from RNA sequencing data. MiXCR looks specifically for sequences associated with T cell receptors (a protein complex found on the surface of T cells).
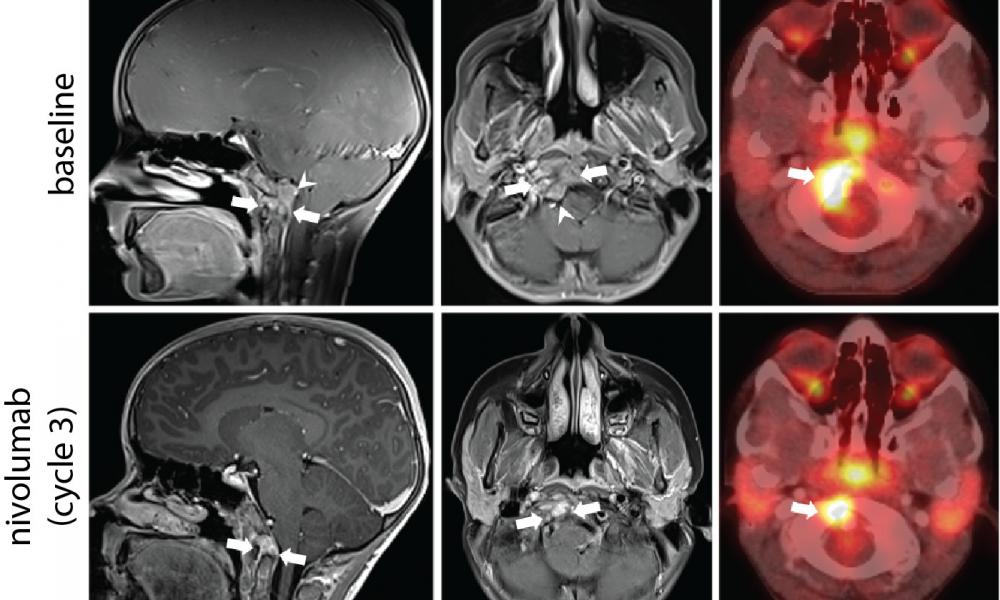
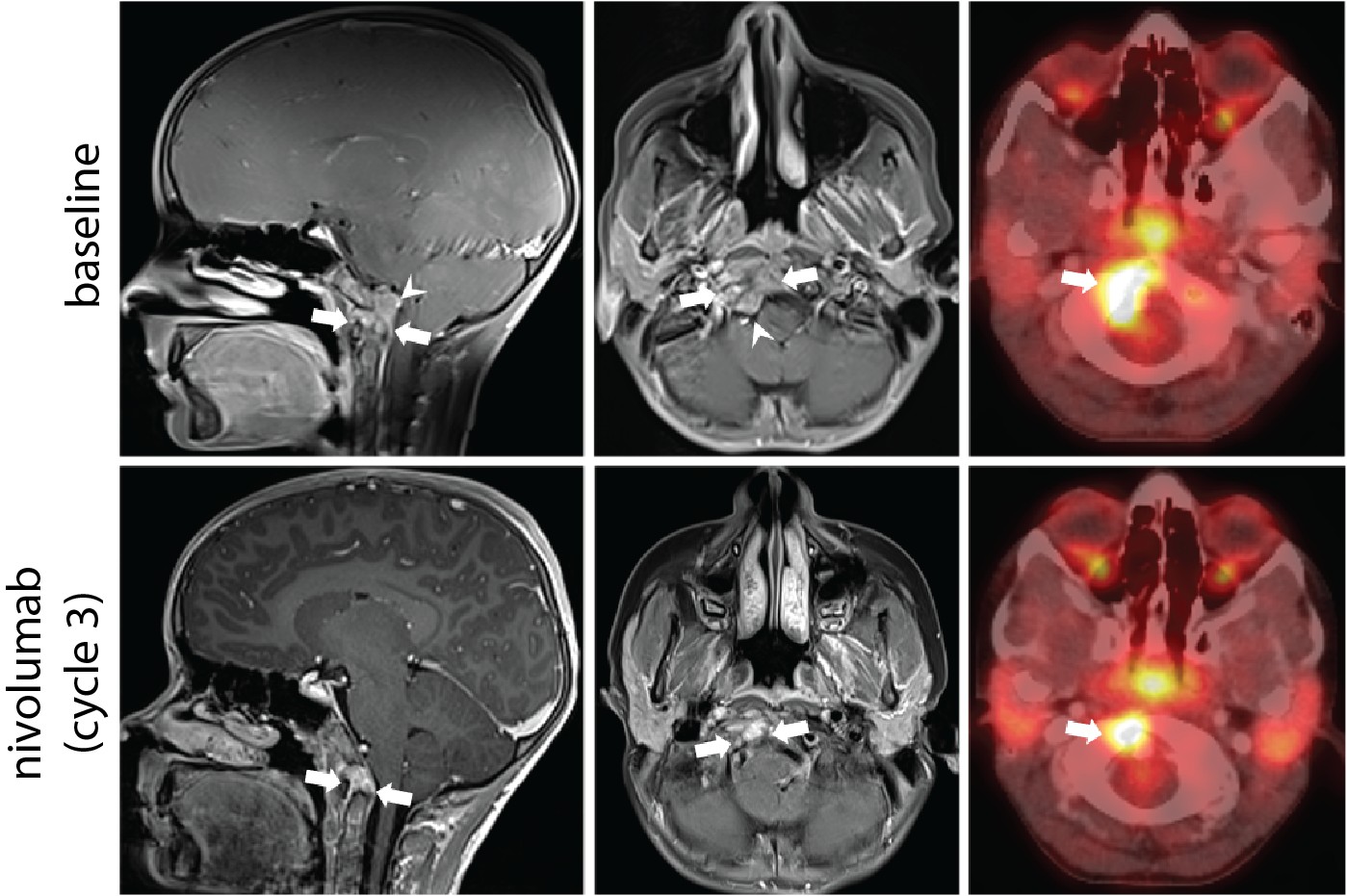
The high level of CD8+ T cells in the immune microenvironment, in addition to the presence of PD-L1 expression in the RNA sequencing data, suggested that Patient A’s tumour was immunologically hot. To confirm, Dr. Brad Nelson's lab at BC Cancer's Deeley Research Centre performed multiplex immunohistochemistry analysis to verify the presence of tumour infiltrating immune cells.
Treatment with an immune checkpoint inhibitor, nivolumab
Based on these results, Patient A was treated with nivolumab, an immune checkpoint inhibitor (ICI). This therapy resulted in a 58% reduction in the tumour size and improved the patient’s quality of life. A series of experiments conducted by the Holt lab discovered that T cells present in the tumour were reactive to brachyury, lending support to the hypothesis that the clinical response following treatment with ICI therapy may have been mediated by recognition of brachyury by the patient’s immune system.
Unfortunately, ICI was not considered as a potential therapy for patient B—who entered POG earlier than the first—because immune cell abundance estimates were not part of POG analysis at that time. CD8+ T cell estimates were measured retrospectively and revealed a similarly high CD8+ T cell score, but the patient passed away from recurrent disease before ICI therapy could be considered.
In her article “Transcriptome analysis reveals key information about a rare pediatric cancer and response to immune checkpoint inhibitor monotherapy”, posted in the Nature Portfolio Cancer Community website, Dr. Laura Williamson, Clinical Informatics Team Lead at the GSC and first author of the paper, writes:
“It is unclear if immune checkpoint inhibitors would have been a suitable option for this patient and we do not know if they would have had a similar clinical response; however, this missed opportunity highlights the power of incorporating new methodology in precision cancer initiatives, and the value of complete, accurate and permanent datasets amenable to retrospective analysis.”
Future application of ICI therapy to other pediatric tumours
Although standard of care for various adult cancers, ICIs are not commonly used to treat pediatric solid tumours—such as pediatric chordoma—because of their low mutation burden. The results of this study, however, suggest that ICIs may be effective for a subset of chordomas that are “immunologically hot”.
Collaborative research, including work done by both the Marra lab and Hirst lab, has also identified a subset of rhabdoid tumours—another INI1-deficient pediatric cancer—that has high levels of tumour-associated CD8+ T cells. Based on the molecular features shared between these rhabdoid tumours and the chordoma cases described in this paper, the authors of the case report suggest that a subset of rare, difficult to treat pediatric malignancies may benefit from ICI therapy.
As Dr. Williamson concludes, “Immunological and genomic profiling will be important for determining which patients may respond to therapy. Establishment of prospective trials that include immune profiling as part of patient selection are required to evaluate the predictive value of these features for patient response.”
Acknowledgements
In the paper, the authors thank patients and families, the POG team, GSC's technical and project management platforms and the generous support of the BC Cancer Foundation, BC Children’s Hospital Foundation, and their donors. They also acknowledge GSC’s Library Construction, Biospecimen, Sequencing, and Bioinformatics and the German Cancer Research Center (DKFZ).
The work was supported by funding from Genome BC, Genome Canada, the Canada Foundation for Innovation (including the CGEn platform), Canada’s Networks of Centres of Excellence, the BC Knowledge Development Fund, Canadian Institutes of Health Research (CIHR) and the Canadian Epigenetics, Epigenomics, Environment and Health Research Consortium (CEEHRC). The results were in part based on analyses of data generated by The Cancer Genome Atlas, Xena Public Data Hubs, Therapeutically Applicable Research to Generate Effective Treatments (TARGET) and the Treehouse Childhood Cancer Initiative.
Image created with BioRender.com
Learn More
Learn more about chordoma.
Learn more about POG.
Citation
Williamson, L.M., Rive, C.M., Di Francesco, D., Titmuss, E., Chun, H. E., Brown, S.D., Milne, K., Pleasance, E., Lee, A.F., Yip, S., Rosenbaum, D.G., Hasselblatt, M., Johann, P.D., Kool, M., Harvey, M., Dix, D., Renouf, D.J., Holt, R.A., Nelson, B.H., Hirst, M., Jones, S.J.M., Laskin, J., Rassekh, S.R., Deyell, R.J., Marra, M.A. Clinical response to nivolumab in an INI1-deficient pediatric chordoma correlates with immunogenic recognition of brachyury. npj Precis. Onc. 5, 103 (2021). https://doi.org/10.1038/s41698-021-00238-4
*bold font indicated members of the GSC.