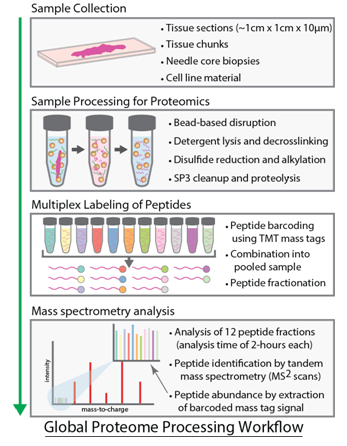
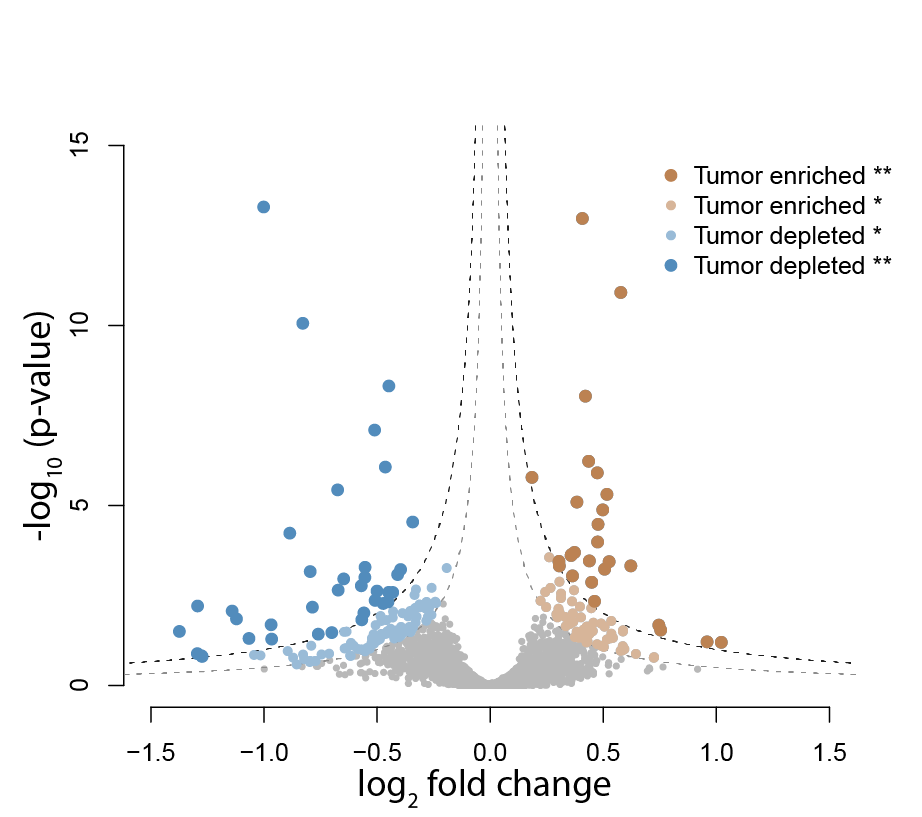
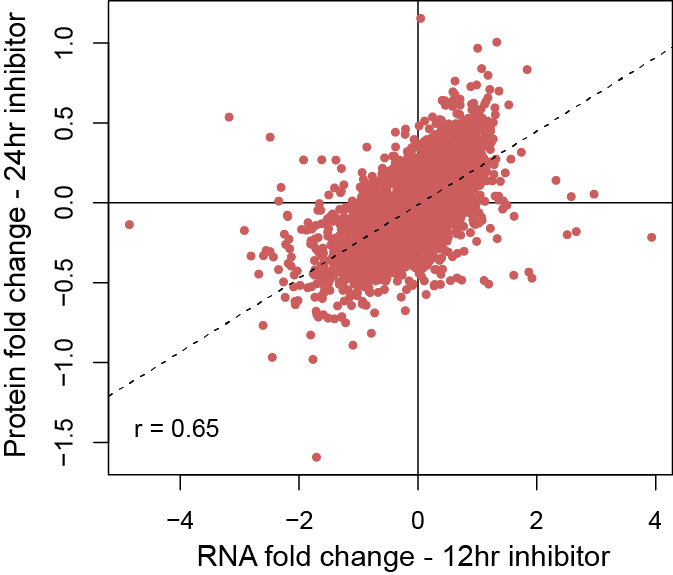
The GSC Proteomics Platform performs collaborative proteomics research to characterize and quantify the changes to proteins and the proteome that drive tumourigenesis. It utilizes state-of-the-art mass spectrometry instrumentation and novel proteomics technologies. The goal is to identify and validate therapeutic targets and biomarkers for the translation of genomics information into clinical practice. The Proteomics Platform supports >23 grants and >50 projects for >30 investigators using field leading mass spectrometry (MS) instrumentation and proteomic technologies.
Technologies:
- global and targeted quantitative proteomic and mass spectrometry (MS) methodologies
- quantitative analysis of clinical (FFPE) patient samples
- analysis of ‘translatome’
- analysis of splicing and aberrant spliced proteins
- discovery and analysis of protein post-translational modifications (PTMs)
- development of isogenic cell culture systems to study aberrant protein function
- recombinant protein expression and purification
- protein chemistry
Charles and Elaine Shnier Mass Spectrometry Instrument Suite:
ThermoFisher Orbitrap Fusion
- global proteome and phospho-proteome analysis
- ideally suited for TMT and SILAC quantification, and identification of post translational modifications.
AB Sciex 5600+ TripleTOF
- discovery analysis and label free quantification using the SWATH technology in the analysis of protein complexes and pathways.
- General protein and proteome identification and analysis by tandem MS.
AB Sciex 6500 QTrap
- targeted quantitation of mutated or splice isoforms of proteins, protein complexes, protein pathways and biomarkers using MRM/SRM.
AB Sciex 4000 QTrap
- targeted metabolite analysis
Protein Engineering, Expression and Biochemistry Suite:
- peptide and protein fractionation (IEF, chromatography)
- protein engineering
- biochemical assays
- density gradient analysis
Collaborative Services
The GSC Proteomics Platform performs collaborative research to characterize and quantify the changes to proteins and the proteome that drive tumourigenesis. The goal is to identify and validate therapeutic targets and biomarkers for the translation of genomics information into clinical practice.
The GSC Proteomics Platform has two primary research areas:
- The functional analysis of gene products (proteins) with aberrant expression in tumours, or identified as somatically mutated through tumour genome sequencing;
- To quantify the global proteome changes in patient tumour samples to identify biomarkers for diagnosis, prognosis, and drug response.