Understanding the basis of acute myeloid leukemia (AML) requires investigating preleukemic syndromes such as bone marrow failure (BMF) and myelodysplastic syndromes (MDS)—rare diseases that can act as precursors to AML. The GSC’s Karsan Lab studies the role of the immune system in such syndromes and in their most recent publication, the researchers have implicated proteins TIRAP, IFNγ, and Hmgb1 in the development of BMF syndrome.
Acute myeloid leukemia and its precursor syndromes
Acute myeloid leukemia (AML), a cancer of the blood and bone marrow (the spongy tissue inside bones where blood cells—red, white, and platelets—are differentiated from stem cells), occurs due to the inappropriate accumulation of stem cells incapable of differentiating into mature blood cells.
Rare diseases such as bone marrow failure (BMF) syndromes, disorders defined by the inability to make enough blood, or myelodysplastic syndromes (MDS), a group of cancers characterized by a lack of mature or healthy blood cells, can act as precursors to AML.
The role of the immune system in the bone marrow microenvironment
In order to fully understand why AML occurs, it is necessary to investigate the bone marrow microenvironment and preleukemic syndromes including the likes of BMF and MDS.
Previous research has implicated the immune system and multiple proteins as playing a role in BMF and MDS development. For example, IFNγ, a small protein (categorized as a cytokine) that responds to inflammation, has been implicated in BMF development. Another category of proteins/peptides known as alarmins—responsible for raising the “alarm” to activate the immune system—have been implicated in MDS. How these immune-related proteins interact and result in preleukemic syndromes is still unclear.
TIRAP implicated in preleukemic syndromes
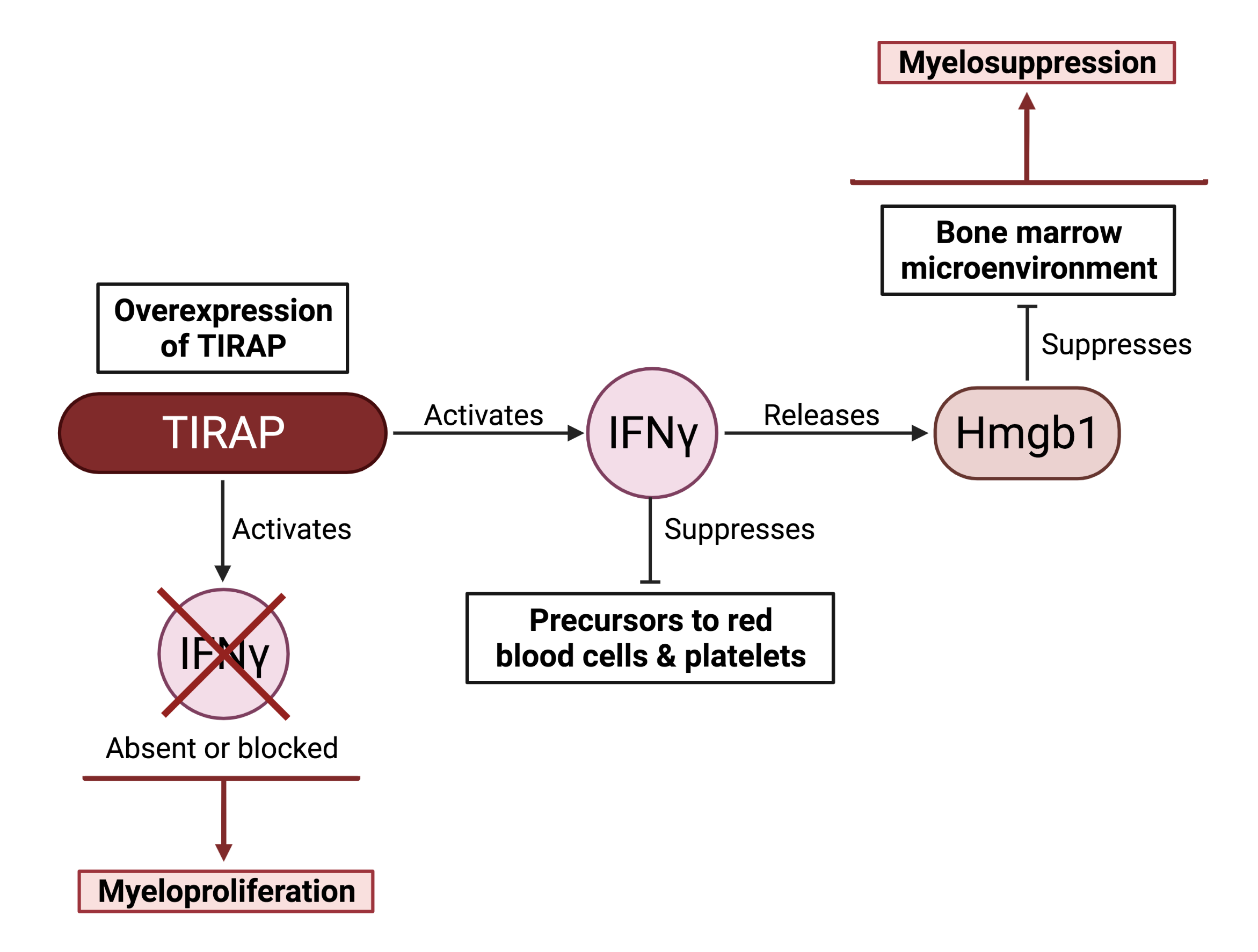
The GSC’s Distinguished Scientist Dr. Aly Karsan and his laboratory recently uncovered a new function for a protein called TIRAP (Toll/interleukin-1 receptor domain containing adaptor protein) in the innate immune response and found that inappropriate TIRAP expression can lead to abnormalities in blood production. In their work, published in the Journal of Experimental Medicine, the researchers found that TIRAP is overexpressed in various MDS patients and, using mouse models, they showed a connection between TIRAP, IFNγ, and an alarmin protein called Hmgb1, in the development of BMF syndrome.
Through a series of experiments, the researchers demonstrated that overexpressed TIRAP induces or “activates” IFNγ. Once induced, IFNγ releases the alarmin protein Hmgb1, which indirectly promotes myelosuppression, the observed phenomenon where the bone marrow does not make enough white blood cells—a characteristic of BMF syndrome. Critically, they also show that in the absence of IFNγ, TIRAP causes myeloproliferation—an overabundance of white blood cells—implying that IFNγ is also needed to suppress the transformation of MDS into AML. Altogether, these results suggest that expression of the TIRAP–IFNγ–Hmgb1 protein axis must be appropriately balanced to avoid both preleukemic disorders and AML.
According to the lead author of the study, Aparna Gopal: “HMGB1 holds potential as a therapeutic target. However, a lot is still unknown about the effects of HMGB1 on blood cell production. Further understanding of all the cells affected by the Ifnγ and Hmgb1 axis within the bone marrow is essential. Most importantly it is crucial to corroborate the findings in human patients.”
Acknowledgements:
This study was supported financially by the Terry Fox Research Institute, the Canadian Institutes of Health Research, and the BC Cancer Foundation.
Image created with BioRender.com. Figure was adapted from a poster abstract, published in the journal, Blood, from the Karsan Lab (Gopal et al. 2021).
Learn more:
Learn more about the ongoing research by the Karsan Lab at the GSC.
Learn more about AML and its treatment options from a recent review article published by the Karsan laboratory (GSC news story available here).
Citation:
Gopal A, Ibrahim R, Fuller M, Umlandt P, Parker J, Tran J, Chang L, Wegrzyn-Woltosz J, Lam J, Li J, Lu M, Karsan A. TIRAP drives myelosuppression through an Ifnγ-Hmgb1 axis that disrupts the endothelial niche in mice. J Exp Med. 2022 Mar 7;219(3):e20200731. doi: 10.1084/jem.20200731. Epub 2022 Jan 28. PMID: 35089323.
*bold font indicates members of the GSC.