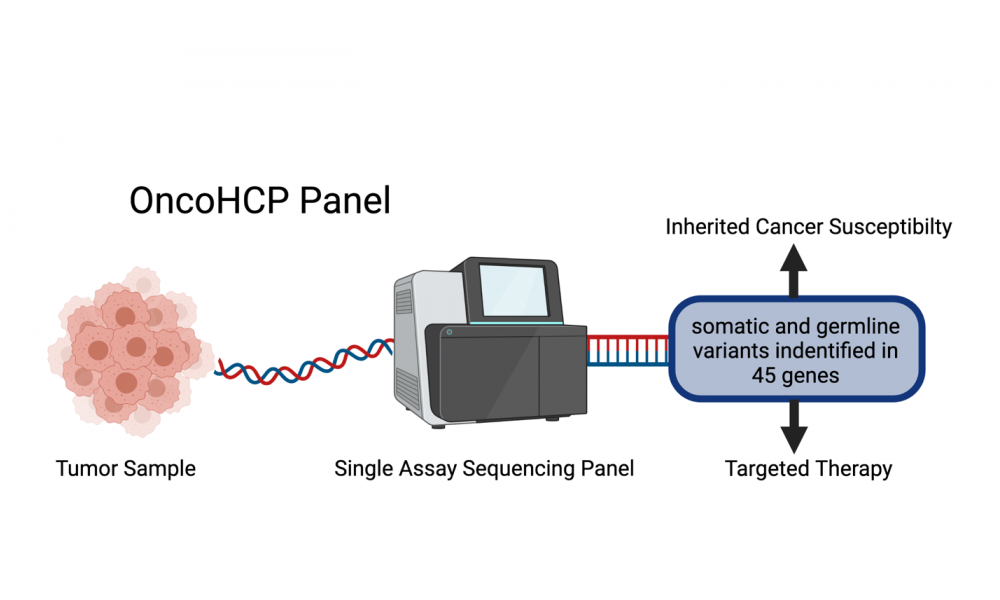
GSC researchers, led by Distinguished Scientist Dr. Aly Karsan, have used next generation sequencing technologies to develop a test that screens tumour tissue for variants in 45 different genes. This includes both acquired and inherited changes known to be clinically significant. In addition to providing potential treatment information to patients with advanced cancer, this new screening tool—referred to as the Oncology and Hereditary Cancer Program (OncoHCP) panel—can identify people with increased susceptibility to cancer.
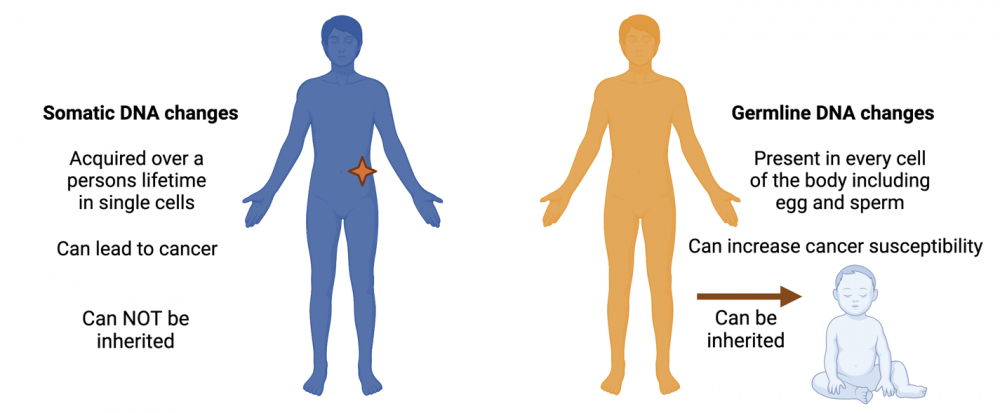
Most cancers are the result of what are called somatic DNA changes that accumulate over time in specific cells of the body. Five to 10 percent of cancers, however, involve DNA changes that are inherited. These changes—often referred to as germline mutations or variants—are present in every cell of the body and make it more likely that a person will develop cancer over their lifetime.
Although testing for germline mutations is available to individuals who meet specific criteria, there are barriers to access for several reasons:
- Uptake for diagnosis of familial cancer syndromes is low.
- Not all individuals with germline mutations meet evidence-based testing guidelines.
- Routine screening of both tumor tissue (to look for somatic changes) and normal tissue (to look for germline changes) is complicated and cost prohibitive.
These limitations are increasingly problematic as more information becomes available on the use of germline data to inform cancer treatment and allow for prevention and early diagnosis in individuals with inherited cancer syndromes, such as hereditary breast and ovarian cancer, Lynch syndrome and Li-Fraumeni syndrome.
The OncoHCP panel
To address this issue, a research team that includes members of the GSC’s Karsan Lab, the Cancer Genetics and Genomics laboratory, and Hereditary Cancer Program and Departments of Pathology at BC Cancer developed the OncoHCP panel. In addition to sequencing the DNA of tumor samples to look for somatic changes, this new test also screened for germline mutations and variants associated with inherited cancer susceptibility.
In their study, published in the Journal of Molecular Diagnostics, 55 samples were screened for somatic and germline variants across 45 genes simultaneously. DNA changes in the genes targeted by the OncoHCP panel are known to be associated with six advanced cancer types and ten cancer susceptibility syndromes. The results of follow up testing showed the panel had excellent sensitivity, specificity, positive predictive value and reproducibility.
Most importantly, detection of somatic variants was not compromised by screening for potential germline variants and cancer susceptibility mutations in the OncoHCP panel. This implies that tumour-only testing could be used to increase both cost-effectiveness and the amount of clinical information obtained through next generation sequencing technologies.

“We’ve shown that sequencing tumours can help detect inherited mutations that predispose for cancer within families,” said Tammy Lau, first author of the study and former Research Programmer in the Karsan Lab. “The OncoHCP assay is a wonderful example of a collaboration between clinicians, genetic counsellors, and researchers that has resulted in a test that helps both patients and their families with their screening and treatment.”
Learn more
- Learn more about testing and counselling for individuals at risk of hereditary cancer.
- Learn more about the Karsan Lab.
- Learn more about research at GSC.
- Learn more about Clinical Sequencing at the GSC.
Citation
Tammy T Y Lau, Christina M May, Zahra J Sefid Dashti, Lucas Swanson, Elizabeth R Starks, Jeremy D K Parker, Richard A Moore, Tracy Tucker, Ian Bosdet, Sean S Young, Jennifer L Santos, Katie Compton, Nili Heidary, Lien Hoang, Kasmintan A Schrader, Sophie Sun, Janice S Kwon, Anna V Tinker, Aly Karsan. Use of Treatment-Focused Tumor Sequencing to Screen for Germline Cancer Predisposition. The Journal Of Molecular Diagnostics.
*bold font indicates members of the GSC.
Funding
This study was supported by Genome British Columbia, the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the John Auston BC Cancer Foundation, and AstraZeneca.