The development of novel therapeutics for the treatment of chemotherapy-resistant prostate cancer relies on disease models that accurately mimic the progression of human disease. When transferred into mice, a particular cell line does just that, allowing researchers to test the efficacy of potential drugs. But studies have shown that the cell line suffers from a lack of reproducibility and that an improved model of treatment-resistant prostate cancer is needed.
In a new study published in the journal Human Cell, led by GSC Distinguished Scientist Dr. Marianne Sadar, researchers have isolated and characterized a pure cell line, improving experimental reproducibility and enhancing confidence in results.
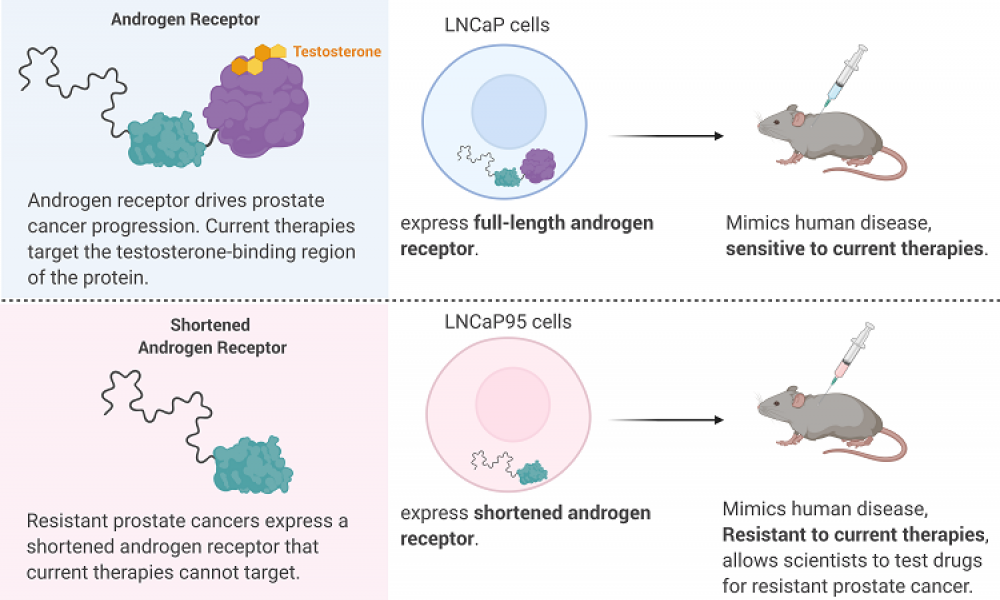
Prostate cancer is driven by a protein called Androgen Receptor (AR) which, when bound to testosterone, drives the expression of a host of genes. The importance of AR in prostate cancer is exemplified by the development of a range of therapies targeting the protein, either through blocking the production of testosterone (androgen deprivation therapy) or by preventing the binding of testosterone to AR (antiandrogens).
While these treatment strategies can slow the progression of prostate cancer for a time, most cases will eventually develop resistance due to the shortening, or truncation, of the AR. Truncated ARs are missing the part of the protein that binds testosterone and antiandrogens, rendering current therapeutics ineffective.
A novel cancer drug developed by Dr. Sadar and her team, ralaniten, targets the AR by binding to a region of the protein that is retained within the truncated form. The drug has shown efficacy in the laboratory against prostate cancer cells expressing both the full-length AR and the truncated form and is currently in clinical trials.
A cell line that accurately mimics the progression of human prostate cancer in an animal model would aid in the optimization of ralaniten and its derivatives. The cell line LNCaP95 was derived from a prostate cancer cell line called LNCaP. Unlike the parental strain, LNCaP95 is resistant to antiandrogen therapies and produces the truncated form of AR, making it an attractive model for the development of alternative prostate cancer drugs. But the irreproducibility of experiments conducted with LNCaP95 led Dr. Sadar’s group to pursue a more robust disease model.
Through serial dilution, her team isolated single cells from the LNCaP95 cell line and allowed them to proliferate. This gave rise to different clones, with each clone consisting of genetically identical cells that arose from one parental cell. Characterization of the purified clones led to the identification of a subclone that is resistant to antiandrogen therapy, expresses the truncated AR and shows enhanced reproducibility in in vivo experiments.
The isolation of an LNCaP95 subclone that proliferates independent of testosterone, is resistant to antiandrogen therapy and sensitive to ralaniten has created a tool for the identification and optimization of novel drugs for treatment-resistant prostate cancer. The enhanced reproducibility improves confidence in experimental results and will enable researchers to better triage drug candidates for clinical trials.
Jacky K. Leung, Teresa Tam, Jun Wang, Marianne D. Sadar. 2020. Isolation and characterization of castration‑resistant prostate cancer LNCaP95 clones. Human Cell. https://doi.org/10.1007/s13577-020-00435-6
This work is supported by a grant from the National Cancer Institute of the National Institutes of Health.
Learn more about research in Dr. Sadar’s lab.
Learn more about research at the GSC.