Researchers have developed a rapid and highly sensitive method for ensuring cell-based cancer therapies are contaminant-free.
Personalized, cell-based cancer therapies are proving to be a life-saving option for patients with otherwise incurable cancers. But producing these therapies is a major bottleneck, and ensuring the products are not contaminated by microorganisms is vitally important. Prior to use, cell-based cancer therapies must be thoroughly screened. This process can take up to a month—time that many patients do not have.
Researchers at Canada’s Michael Smith Genome Sciences Centre (GSC) at BC Cancer, led by Dr. Robert Holt, have developed a new method for the detection of contaminants, enabling the high-confidence screening of cell-based cancer therapies within just eight hours.
Production of cell-based therapies
A promising new type of cell-based cancer treatment called CAR T-cell therapy exploits the power of the immune system to fight cancer. A patient’s T cells—key players of our immune systems—are taken from the body, trained to detect and eliminate cancerous cells, allowed to replicate in the laboratory to create an army of cancer-fighting cells, and then injected back into the patient where they carry out their search-and-destroy functions. While there is mounting evidence that these “one-time only” treatments are highly effective, the expense and complexity of production remains a bottleneck.
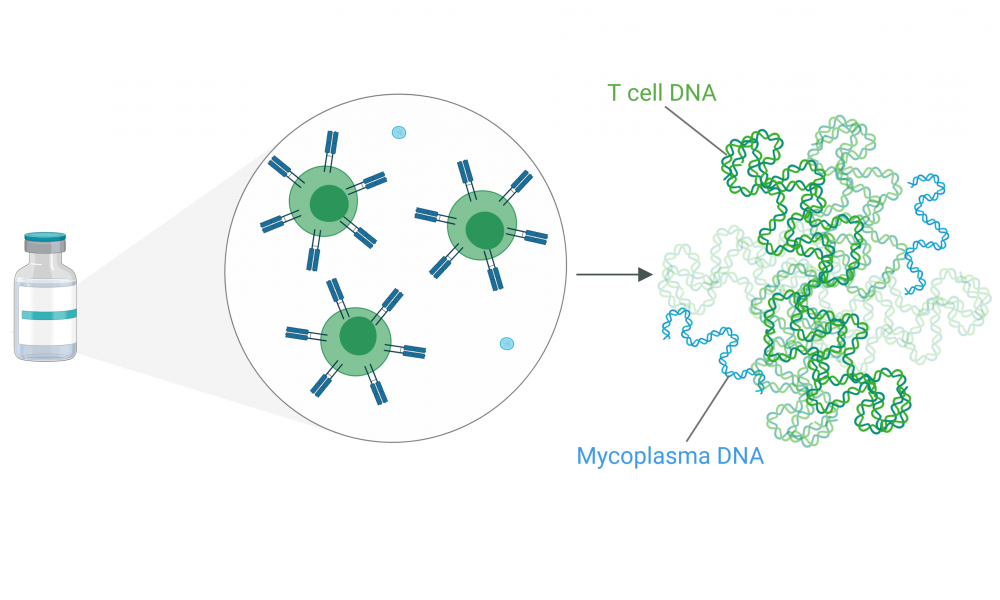
To grow in a laboratory, T cells require a rich source of nutrients and a warm environment—conditions that are unfortunately ideal for the growth of bacteria. Many precautions are taken during production of cell-based therapies, so contamination is rare. But when it does occur, a particular type of bacteria called Mycoplasma is often the culprit.
Mycoplasma contamination
“Mycoplasma is commonly found in the throats and nasal passages of healthy people, so even talking near the samples is a potential source of contamination’” said Lisa Dreolini, Research Assistant at the GSC and first author of the study, “This is why Health Canada requires that every sample be confirmed as Mycoplasma-free before being infused into patients”.
Mycoplasma are the smallest known free-living organisms and are considered to be the simplest form of bacteria. As regular inhabitants of the upper respiratory tract, they do not typically cause disease in healthy individuals. But in cancer patients with weakened immune systems, these microbes can cause potentially severe infections.
“Mycoplasma does not pose significant risk to healthy people,” said Dreolini, “But patients being treated with immunotherapy have weakened immune systems because a) they have cancer, and b) their immune systems need to be intentionally depleted prior to receiving this type of therapy”.
Prior methods for screening cell-based therapy products rely on replication of contaminating bacteria so that they can be seen under a microscope—a process that can take up to a month. The method, developed in the Holt lab and published in Molecular Therapy: Methods & Clinical Development, can be done in a day. Rather than looking for the bacterial cells themselves, they treat the samples like a crime scene, looking instead for DNA evidence that the bacteria are present.
Using DNA as an indicator instead of live bacteria is a big advantage. It circumvents the need for growing Mycoplasma bacteria within the laboratory, reducing the amount of time needed to screen the samples while also eliminating a potential source of contamination.
Method validation
Similar DNA-based screening methods have been developed elsewhere, but to be used on patient samples the methods must meet specific criteria as determined by Health Canada.
“The validation process is long and arduous,” said Dreolini, “The detection limit is very important. Health Canada has a specific requirement that you demonstrate that you can detect as little as ten bacterial cells in one millilitre of sample, and you need to be able to do this repeatedly and reproducibly with samples that may vary in composition”.
The Holt lab set out to develop a method with the required sensitivity, robustness and specificity for validation, demonstrating they were able to meet the detection limit reproducibly on a variety of different samples. To put the sensitivity of this method into perspective, finding ten Mycoplasma cells in one millilitre of sample is similar to finding ten particles of sand floating in fifteen Olympic sized swimming pools, and doing it within eight hours.
Sharing their method with the scientific community will enable rapid and cost-effective screening of cell-based cancer therapies across the country, facilitating efficient delivery of these life-saving therapies to patients enrolled in clinical trials.